Sustaining the Pace

Three perspectives on how sustainability is being achieved in the medical device market
Summary
- The quest for credible sustainability and environmental protection is now mainstream in society and has become a common goal for medical device companies
- However, a tension exists between the desire to recycle/re-use and the culture of single-use disposable devices which is designed to protect patient and healthcare worker safety
- Nevertheless, sustainable targets must be introduced to reduce the immense amount of waste and energy consumption for which the healthcare sector is responsible – much higher than most other sectors
- In many instances, sustainability initiatives can be seen to have a strong business case – saving money as well as reducing emissions
- Sustainability programmes in the medical industry are on the rise and cover three main areas, as outlined in this short paper:-
- Recycling
- Optimizing manufacturing processes
- Sustainability by design
Sustainability becomes the norm
Environmental responsibility and stewardship is rapidly becoming a normalised, ‘must do’ function within the corporationi. At the individual level, many of us are asking questions about how we shop greener and make conscious choices to reduce our impact on the natural environment. What twenty years ago was a relatively niche concern is now a mainstream requirement.
The medical device industry is also becoming subject to pressure from regulators, hospital systems, governments, and consumers alike to conform to this emerging reality. Nevertheless, conformity comes at a very real cost; at the same time, there is a widespread feeling that there is a very real risk of losing access to markets around the world for medical device companies who do not take the initiative to become more environmentally responsible and sustainable.
Tensions for medical products
The essential tension over medical products lies in the relationship between sustainability and infection control, an issue that could not be more topical as the world struggles out of a pandemic situation. Indeed, while the immediate issue is one of personal protective equipment (PPE) disposal, one commentator notes “how the disruption caused by COVID-19 can act as a catalyst for short-term and long-term changes in plastic waste management practices throughout the worldii.” We all want to reduce waste in the healthcare system; but at the same time, we all expect to be kept as safe as possible while undergoing medical treatment. According to the European Centre for Disease Prevention and Control, Healthcare Acquired Infection rates run at between 5% and 8% of patients in most developed countriesiii. This is still unacceptably high and remains under threat from the spread of antimicrobial resistance, notes the Centers for Disease Control in the United Statesiv, which also tells us that more than 2.8 million antibiotic-resistant infections occur in the US each year, and more than 35,000 people die as a result. This issue, along with concerns over avoidable healthcare worker infections resulting from needlestick injury, have led to a strict single-use, disposable culture around many invasive medical devices, along with mandatory legislation in the US and Europe. The tension between patient safety and environmental responsibility is therefore a challenging path to tread.
The size of the prize
There can, however, be no doubt about the size of the environmental problem in healthcare. The US healthcare system contributes 10% of the nation’s carbon emissions and 9% of harmful non-greenhouse air pollutantsv. Moreover, its rate of greenhouse gas emissions increased 30% between 2006 and 2016vi. The healthcare sectors of the United States, Australia, Canada, and England combined emit an estimated 748 million metric tons of greenhouse gases each year, an output greater than the carbon emissions of all but six nations worldwidevii. In Europe, legislation has been enacted over the last twenty years to manage the situation. European standards such as the Waste Electrical and Electronic Equipment (WEEE); Restriction on Hazardous Substances (RoHS); Registration, Evaluation, and Authorization of Chemicals (REACH); and the Energy Using Products (EuP) regulations have significantly altered the manufacturing processes, specific labelling, compliance with disposal restrictions, and creation of instructions for end-of-life management and recycling. Although many medical devices are currently exempt from these regulations, several directives, including RoHS and WEEE, are in the process of being reviewed and could be applicable in future. In addition, many devices are becoming more ‘connected’ in our increasingly digitalised world, which then brings them under the regulatory purview of authorities that govern devices with electronic components. Europe remains a key market for many medical device manufacturers, and so in the United States, pressure from a nondomestic customer base has already forced many US companies to comply with WEEE and RoHS. In addition, many commentators are now saying they regard the passage of strict environmental regulation and/or legislation in the United States as inevitable.
The emerging business case for sustainability
Much of the US healthcare system, including large hospitals and group purchasing organizations (GPOs), have started to recognise that sustainable purchasing practices can actually reduce costs over time. It is important that good citizenship can find a compelling business case, as the combination of the two factors has been seen historically to move such policies into mainstream practice. Evidence of real action on this front is embodied in the fact that many GPOs have appointed and empowered Senior Directors of Environmentally Preferred Sourcing who are successfully implementing the sustainable purchasing business caseviii.
Incineration of medical waste has been a traditional means to reduce the volume of waste and destroy biohazardous materials. However, this process produces environmental contamination by releasing nitrous oxide, as well as known carcinogens including polychlorinated biphenyls, furans and dioxinsix. Exposure to these compounds has been linked with damage to foetal and adult body function as well as the acidification of land and ocean. Moving incineration to recycling therefore has a wide societal impact and reduces damage to people and the environment which ultimately has a tangible cost for society. Increasing recycling rates – especially for plastics – is therefore greatly desirable, and where this is not possible, European regulation sets strict emission limits for incinerators for clinical wastex. Sophisticated filtering systems are also being installed in incinerators to prevent toxic fumes from polluting the atmosphere.
Barriers to sustainability
The situation that the medical device industry faces, then, is that approximately 90% of medical device waste consists of disposable, one-time-use products or components. The simplistic view is to imagine that one can reduce the number of disposable components. However, as we have already remarked, safety standards often prevent this. In addition, the industry’s business model needs to be taken into account to make transition commercially sustainable and ensure security of product supply. Many manufacturers generate the bulk of their revenue from the sale of disposable products or components. Adherence to this business model is underscored by the risks associated with hazardous medical waste, biological contamination, and the high cost of product sterilization and reprocessing.
Sterilization – which could also seem a simple route to product re-use – is often environmentally unsustainable. This is the case even with larger equipment. Take an example from the biopharma industry. A study in BioProcess International found the energy consumption of a stainless steel pharmaceutical powder handling machine, after factoring in cleaning and sterilisation, reached 8,018 megajoules (MJ) of energy. In contrast, the process of manufacturing and disposing of single-use powder handling devices reached 4,156MJ. This means that the disposable set up was ultimately better for the environment despite the resulting waste due to the sheer energy required to maintain the reusable system. Specifically in the healthcare scene, several established sterilization methods, such as the use of glutaraldehyde and ethylene oxide, are not only harmful to the environment but also tend to be regulated by strict disposal rules. As a result, many hospitals and medical device companies are adopting less toxic methods such as hydrogen plasmaxi.
Toby Cowe, Technology Development Group Manager R&D, Owen Mumford
Sustainability trends in the medical device industry
Although the obstacles to sustainable design in the medical device industry may seem considerable, much can still be achieved. And it is likely that the medical device industry will be challenged by governments and regulatory authorities to square the circle between product sustainability, patient safety standards and commercial viability. Many medical device firms have started to establish their environmental record and publish it to position their progress. Evidence is emerging of the regular inclusion of environmental credentials in medical device tendersxii. As the World Health Organisation has noted, “using health systems’ buying power to maximize positive environmental outcomes, recognizing that influencing suppliers to factor environmental impacts into their manufacturing processes is a powerful lever for significant changexiii.”
What then are the key aspects of sustainability that leading medical device manufacturers are putting into practice, in order to deliver a sustainability contribution while also complying with all the regulatory structures that safeguard patients and healthcare professionals? We have summarised them under three key headings here.
Recyclability
There are two issues at stake under the heading of recyclability. One is whether a device can safely and effectively be re-used. The other is whether its materials can be reprocessed and re-used, whether in the medical or another context. Device re-use is – as we have already discussed – fraught with safety concerns and questions over the overall environmental impact of sterilisation. The Medical Device regulation, along with local regulation from country authorities such as the MHRA, makes it quite clear that a recycled device has to meet all the criteria and approval strictures that a brand new device has to go through. There will no doubt be further developments though. In August 2019 the European Commission closed its feedback period on safety and performance requirements for single-use device reprocessing, and there will certainly be outputs resulting from this consultation when pandemic aftermath management priorities have been covered.
In terms of recyclable materials, much can be achieved. PVC itself can be recycled several times without losing its critical properties. Equally, work has been done around the use of more easily recyclable plastics, such as renewable polyethylene and polyethylene terephthalate (PET). To work in practice, closed loop recycling schemes have to be put in place which have a programme to recover the waste materials from hospitals or patients and bring them back into the recycling process. Although complex to implement the gains from such schemes would be very considerable indeed. There is an estimated one million tons of clean, non-infectious healthcare plastic waste generated in healthcare facilities every year according to the Healthcare Plastics Recycling Councilxiv. Nor does the science of recycling stand still. Monomer extraction techniques in development enable recycled polymers to be broken down to their constituent monomers. If ubiquitously adopted, this could mean a virtually limitless recyclability of some polymers without loss in performancexv.
Packaging plays a strong part in the recycling process. In some instances, packaging manufacturers are decreasing packaging volume by favouring sealed trays instead of pouches. In addition, some are reducing the number of components required in the overall package. For instance, this could be achieved by laser-etching instructions directly on to the tray, where regulation permitsxvi. Others are exploring the use of faster degrading plasticsxvii. And incorporating logistical requirements right at the beginning of the packaging design process can help specify optimum transport that helps reduce energy consumption – especially where, for instance, cold chain storage is requiredxviii.
Sustainability Changes Implemented to Pharmaceutical Packaging Materials & Design
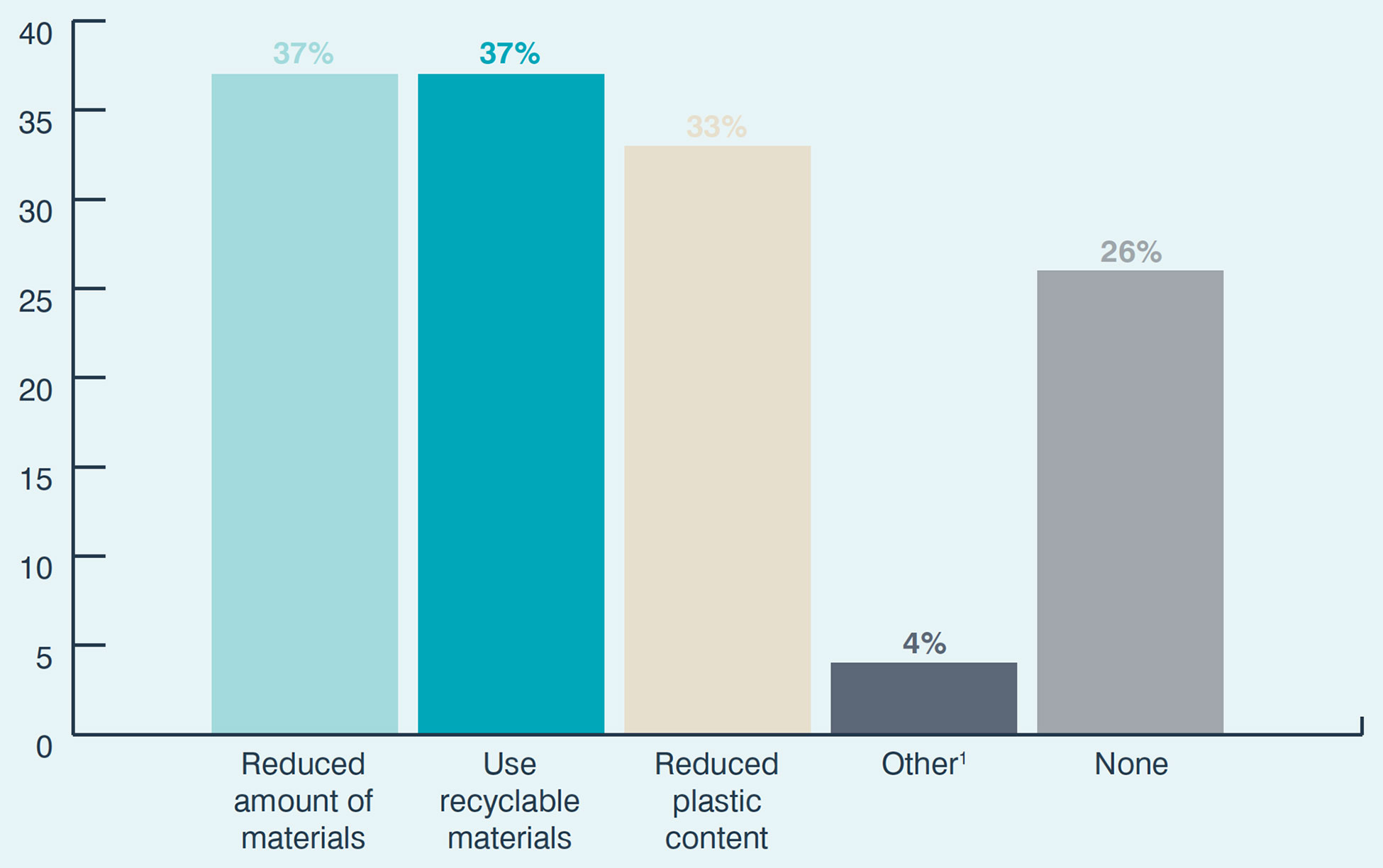
Question: What changes have you already implemented to pharmaceutical packaging design and materials to provide a more sustainable alternative? (Please select all that apply)
Base: All respondents; multiple answers permitted (n=166). 1Other includes: Improved Nonreactive Materials; More Reusable And Biodegradable Components; New Way To Administer; And Training On Critical Thinking About Sustainability With Several Groups Of Health Professionals
Owen Mumford Pharmaceutical Services, in partnership with Pharma Intelligence, Injectable Combination Products. August 2020.
Sustainable Manufacturing
Medical device manufacturers can achieve much in the sustainable development of their manufacturing processes, all of which has a positive environmental impact which the company can present to the procurement chain. Clearly, reduced use of water, energy efficiency, logistics optimization and reduced use of polluting chemicals are all factors that manufacturers are attending toxix. Manufacturers are collaborating with healthcare providers to scope out, report and measure such improvements via organisations such as the UK’s Sustainable Healthcare Coalition, as well a co-operative work on environmentally sustainable patient pathways in the health service itself.
Energy-efficiency is an important factor in making medical device manufacturing more sustainable, not only because it has a direct impact on CO₂ emissions, but because it leads to immediate cost savings. Overall sustainability improvement initiatives often harness future savings from energy cost reductions to finance the whole programme.
Overall productivity, achieved through the adoption of new generation manufacturing technology, may not only be more energy efficient, but can also reduce waste and shorten time to market. A good example is the adoption of 3D printing to develop and test prototypes, possibly developing the optimum product mould more quickly, fulfilling both human factors usability testing but also refining production parameters to minimise raw materials volumes and maximise output productivity. Another example is the adoption of ‘digital twin’ production software, that uses inline sensors to create a virtual, real-time mirror of the production environment. This allows production line refinements to be developed, tested and implemented, all without interrupting actual physical production. The goal here is to get as near as possible to ‘zero defect’ manufacturing, again reducing waste.
Toby Cowe, Technology Development Group Manager R&D, Owen Mumford
Sustainability by Design
Many of the key principles of sustainable product design start by understanding and developing a product life cycle, not just a product – considering concept development, material selection, design and engineering, manufacturing, packaging, transportation, sales, use, and end-of-life disposal, right from the start. This approach is already deployed for manufacturing efficiency, time to market, risk reduction, safety and regulatory compliance, and packaging and transportation costs. It is simply a case of extending existing disciplines to also evaluate energy efficiency, environmental impact, material usage, and recycling. In some aspects, existing FDA and EMA quality system requirements touch on these environmental considerations – particularly around tracking, materials safety/efficacy and disposal. Similarly, LEAN manufacturing methodologies seek to reduce inefficiency in several key related areas, such as overproduction, waiting time, transportation, processing, inventory, motion, and scrap.
Toby Cowe, Technology Development Group Manager R&D, Owen Mumford
A number of other more specific sustainability considerations can be built into product design and engineering. For example: ease of disassembly will have a major impact on recycling costs and methods; device size optimisation, device simplification and packaging reduction will reduce waste and transport impact; and harmonising raw materials and production methods between different products will save on cost and waste, while also opening the possibility of greater agility of production tasks between production lines. For disposable products, choosing materials that limit environmental damage during disposal and incineration can reduce toxic air emissions and reduce waste processing costs.
Toby Cowe, Technology Development Group Manager R&D, Owen Mumford
The current reality is that most devices – especially parenteral or other invasive products – will have to retain a disposable element to meet regulatory safety and hygiene requirements. However, the art is to design a minimum disposable unit within a reliably re-usable ‘shell’. As devices rapidly become more digitally connected (delivering stupendous therapy and cost benefits for remote patient management and safe self-administration) commercial considerations have to drive developments in this direction. The costs of disposable electronics would simply not be viable, and – as we have previously noted – would not be acceptable in the light of electronics disposal regulations. All the design science then has to be focused on creating a simple, repeatable interface between the two component sections so as not to impair the functionality or efficacy.
For more information please contact pharmaservices@owenmumford.com
i. See, for instance: Medical Product Outsourcing, The Challenge and Opportunity in Corporate Environmental Sustainability Efforts, 4 Jan 2020; Journal of Cleaner Production, Exploratory study of the state of environmentally conscious design in the medical device industry, 1 Dec 2015;
ii. Renewable and sustainable Energy Reviews, J.J.Klemes, Y.V.Fan, R.R.Tan, P.Jiang, Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19, 27 Apr 2020
iii. https://www.ecdc.europa.eu/en/healthcare-associated-infections-acute-care-hospitals/database/prevalence-hais-and-antimicrobial-use/observed
iv. CDC Antibiotic Resistance Threats in the United States, 2019
v. American Journal of Public Health, M.J.Eckelman, J.D.Sherman, Estimated Global Disease Burden of US Health Care Sector Greenhouse Gas Emissions, Apr 2018,Ref. Am J Public Health. 2018 April; 108(Suppl 2): S120–S122
vi. ibid
vii. ibid
viii. Healthcare Plastics Recycling Council, Environmentally Preferred Sourcing Powers Up Sustainability in Healthcare, 19 Dec 2018
ix. Environment International, Vol 132, M.Ansari, M.H.Ehrampoush, M.Farzadkia, E.Ahmadi, Dynamic assessment of economic and environmental performance index and generation, composition, environmental and human health risks of hospital solid waste in developing countries; A state of the art of review, Nov 2019
x. Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the incineration of waste
xi. Medical Product Outsourcing, Coming Clean, 6 Apr 2020; MD+DI, Sustainability in Medical Device Design, 1 Sep 2008
xii. See, for instance: Southern Health NHS Trust, Sustainable Procurement Procedure, 3 Sep 2018; McKinsey, Value-based procurement in European medtech, 6 Dec 2018; Plastics Today, Kaiser Permanente uses advanced sourcing strategies to eliminate DHP, cut costs, 27 Nov 2013;
xiii. WHO, Environmentally sustainable health systems, 2017
xiv. HPRC, Why recyclers should consider healthcare plastics as an important feedstock, 23 Jan 2019
xv. Nature Reviews Materials 5, 501-516(2020), G.W.Coates, Y.D.Y.L.Getzler, Chemical recycling to monoer for an ideal, circular polymer economy, 14 Apr 2020
xvi. MedTech Innovation, Going Green, 22 Feb 2019
xvii. Manufacturing Chemist, Sustainability and innovation in the complex combination therapy sector, 26 Mar 2020
xviii. Team Consulting, Sustainability in the medical devices sector, 2020
xix. Medical Technology, Making sustainable medical devices, 10 Jul 2019








