UniSafe® Whitepaper
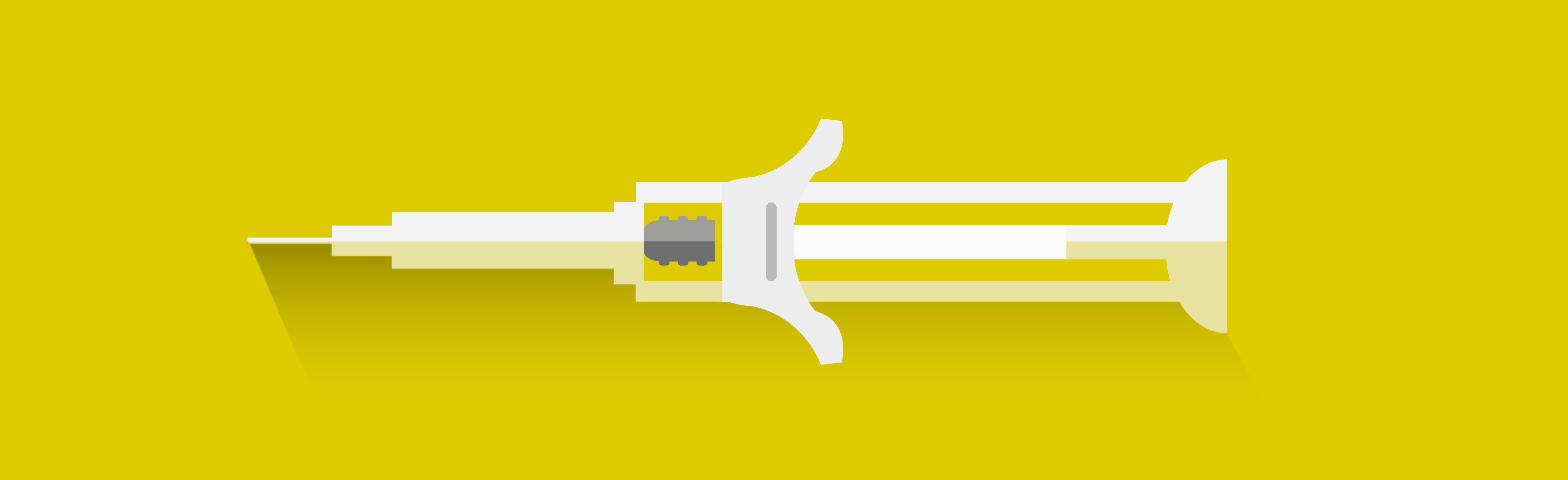
UniSafe® is a passive, springless safety device for pre-fillable syringes. Using a patient centric approach to optimise confidence in medical device design.
Introduction
For pre-filled safety devices, the market is set to triple by 2020, from US$250 billion (£205 billion) to $797 billion, and between 2017 and 2018 alone the sales forecast is set to see its largest increase of 34% annual growth1 .
Pharmaceutical companies and healthcare providers alike are facing a change in market demand. The safety and confidence of the patient must be at the centre of an effective treatment delivery.
Regulations in recent years have also demanded this and safety injection devices have emerged as one of the key focus areas for device solutions. Not only do these enhance the patient experience and increase a wider adoption of treatment (due to a safer and more effective experience) but they also encourage long term economic benefits to the healthcare system by allowing the patient to treat themselves effectively.
Government regulations and end-user demand have become two strong driving factors behind device development amongst medical device teams. In response, there have been huge advances in innovation and technology surrounding the design and use of safety devices.
Background
The current market place is largely composed of retractable and non-retractable safety syringes which are activated through a spring component. This has previously led to challenges, including accidental activation where a device may activate in transit or under-dosing, where it may be challenging for the user to visually confirm the full dose has been delivered (for instance, if a spring is placed at the front of the syringe barrel, obstructing the view.)
Scenarios such as these can impact the efficacy of the treatment – the patient may not receive the correct dosage, the device may be under-populated and there may be discomfort experienced in using the device, alongside the risk of injury. These factors create a barrier to continued treatment.
Utilising a thorough understanding of long-term treatment and injecting, partnered with world class research, design expertise and engineering capabilities, Owen Mumford Pharmaceutical Services has responded to the market need for a safer, more effective syringe device.
UniSafe® is designed to increase patient confidence when injecting, eliminating the possible risks and challenges of traditional sprung syringes.
Evaluation objective
The design of UniSafe® must ensure a patient focused, safety centric experience whilst reducing potential barriers to treatment (painful administration, bulky design, uncomfortable use).
The objective is to determine whether the design of UniSafe® offers the patient or enduser a comfortable, safe and effective treatment experience. METHODOLOGY A study was conducted, comprising of nurses (50) and patients (57), accessing and administrating injections across various conditions in Rheumatology, Oncology, Respiratory, Cardiology and Gastroenterology. All were invited to interact with the device design. The study was conducted with an independent research house2.
Methodology
A study was conducted, comprising of nurses (50) and patients (57), accessing and administrating injections across various conditions in Rheumatology, Oncology, Respiratory, Cardiology and Gastroenterology. All were invited to interact with the device design. The study was conducted with an independent research house2.
Results and discussion
The feedback of the study concluded that UniSafe® was considered intuitive to use, leading to a high confidence rate in drug delivery.
The following key aspects of the device design played an important part in this:
- UniSafe® has been developed with an unobscured pre-filled syringe allowing the user to view the drug and labelling without having to rotate the syringe barrel, reducing the risk of under-dosing. In the study, 94 percent of nurses agreed it was easy to view medication in UniSafe® before delivering the dose, whilst 89 percent of patients stated UniSafe® made it is easy to know that all the dose had been delivered.
- In addition, the device has been built with a strong grip, promoting confidence that the end-user or healthcare provider can administer the treatment safely. For 88 percent of nurses questioned and over 75 percent of patients, grip was a key factor in the design of UniSafe®, particularly in raising confidence that the device would not slip during administration.
- UniSafe® has been designed with a larger, ergonomic plunger head and a smoother, more integrated finger flange for a more comfortable and integrated look and feel. This ensures the end-user can use the device confidently and intuitively, regardless of hand size, dexterity or condition. The device is thus suitable for all patient types and in the study, 82 percent of nurses agreed their patients would find UniSafe® intuitive to use, whilst 83 percent of patients agreed they would feel more confident using the device.
Conclusion
The study highlights how the design of UniSafe® has responded to the needs of the patient first, offering a safe and comfortable experience. By following a ‘patient centric approach’ the device design is both intuitive and easy to use and offers additional safety for the end-user.
Patient centric design is always changing and Owen Mumford Pharmaceutical Services is working closely with pharmaceutical partners to adapt products to meet the ever-growing demand for injectable treatments.
The safety syringe market is set to expand at 9.7 percent Compound Annual Growth Rate (CAGR) following a rise in health and safety awareness between the years 2013 – 20193. Manufacturers utilising a patient centric design are responding to this, changing in line with market evolution and customer demand. The demand and development of injectable devices is a constant feedback loop between end-user and manufacturer and development is vital to both encourage adherence to treatment regimes and help patients effectively self-manage their condition.
With up to 16 billion injections occurring every year, there is a demand for manufacturers to provide devices that are intuitive, safe and easy to use, for both patients and healthcare providers.
Contact details
pharmaservices@owenmumford.com
www.ompharmaservices.com
References
1. Roots Analysis, “Needle stick safety injection devices market 2014 -2024”
2. UniSafe® Safety Device End-User Study – November 16
3. Transparency Market Research “Safety Syringe Market to Expand at 9.7% CAGR Due to Growing Health Safety Awareness and Technological Advancements” (Available at: http://www.transparencymarketresearch.com/ pressrelease/safety-syringes-market.htm) CONTACT DETAILS pharmaservices@owenmumford.com www.ompharmaservices.com








